Prostate Cancer Diagnostics Market Dynamics and Competitive Landscape By 2027
Prostate Cancer Diagnostics Industry Overview
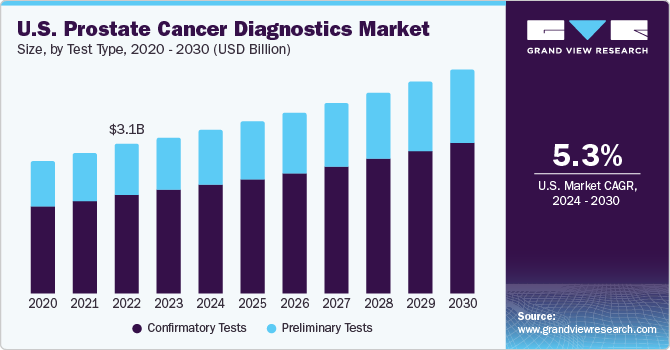
The global prostate cancer diagnostics market size is expected to reach USD 7.65 billion by 2027, registering a CAGR of 13.2% during the forecast period, according to a new report by Grand View Research, Inc. Major drivers include increasing government initiatives, high prevalence of prostate cancer, and technological advancements in confirmatory diagnostic tests.
Prostate Cancer Diagnostics Market Segmentation
Grand View Research has segmented the global prostate cancer diagnostics market report on the basis of test type and region:
Based on the Test Type Insights, the market is segmented into Preliminary Tests and Confirmatory Tests.
- Preliminary test segment is expected to hold the largest market share over the forecast period, owing to a rise in prevalence of prostate cancer. According to the American Institute for Cancer Research, easy availability of PSA screening has facilitated early diagnosis and treatment, increasing survival rates. PSA tests detect unrecognized and small tumors that may or may not develop into an advanced stage of cancer. As these tests facilitate detection before major symptoms appear, they are recommended to most men over the age of 50 and those at risk of developing the disease.
- Rise in product approvals by the Food and Drug Administration (FDA) is anticipated to boost market growth during the forecast period. For instance, in October 2019, Cleveland Diagnostics Inc. received FDA Breakthrough Device Designation for its product IsoPSA Assay, a novel non-invasive blood based diagnostic assay for the detection of the disease.
- Other preliminary tests include the Digital Rectal Exam (DRE) and biomarker tests. DRE tests are used for detecting hard, lumpy as well as the abnormal growth of the prostate gland. Since these tests depend on the skills and abilities of the technician, the results obtained are not accurate and, in most instances, require further tests such as PSA and biopsy. Biomarkers aid in distinguishing between significant and insignificant cancers and the identification of aggressive cancers among men undergoing surgery. These facts are indicative of the rising awareness among general population, facilitating early detection and treatment, propelling the market growth.
- Confirmatory tests include PCA3 test, Transrectal Ultrasound (TRUS), and biopsy. Post preliminary testing, if results of PSA and DRE are abnormal, patients are required to undergo confirmatory tests for further diagnosis. With rising disease prevalence, there has been an increase in the usage of confirmatory tests, which is anticipated to boost the market.
Prostate Cancer Diagnostics Regional Outlook
- North America
- Europe
- Asia Pacific
- Latin America
- Middle East & Africa (MEA)
Key Companies Profile & Market Share Insights
For instance, in 2017, Roche launched a new cancer biomarker, the rabbit monoclonal primary antibody anti-p504s (SP116) for the disease diagnosis. The biomarker is used for detecting α-methylacyl-CoA racemase in tissue sections, enabling easy identification of morphologically different samples such as cancerous, benign, and atypical from a single slide.
Some prominent players in the global prostate cancer diagnostics market include
- MDx Health
- Myriad Genetics, Inc.
- Abbott Laboratories
- Hoffman-La Roche AG
- Siemens Healthineers AG
- OPKO Health, Inc.
- Genomic Health
Order a free sample PDF of the Prostate Cancer Diagnostics Market Intelligence Study, published by Grand View Research.
Comments
Post a Comment