In-vitro Toxicology Testing Market Growth and Competitive Analysis with Key Players 2028
In-vitro Toxicology Testing Industry Overview
The global in-vitro toxicology testing market size is expected to reach USD 51.1 billion by 2028, according to a new report by Grand View Research, Inc. The market is expected to expand at a CAGR of 10.7% from 2021 to 2028. In recent times, the validation and acceptance of in-vitro and alternative testing methods by regulatory agencies are increasing at lucrative pace. Also, ongoing technological advancements to replace the use of animals for toxicology testing purposes have spurred the use of in vitro testing models, in turn, driving the market.
In-vitro Toxicology Testing Market Segmentation
Grand View Research has segmented the global in-vitro toxicology testing market on the basis of end-use, technology, method, application, and region:
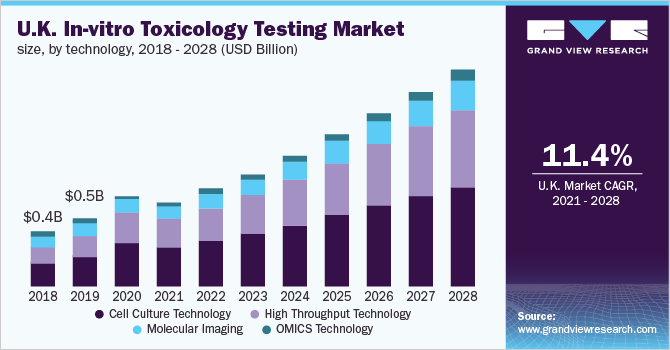
Based on the Technology Insights, the market is segmented into Cell Culture Technology, High Throughput Technology, Molecular Imaging and OMICS Technology
- The cell culture technology dominated the market for in-vitro toxicology testing in 2020 with a revenue share of over 47.0%. It serves as an excellent model for examining toxic effects since it provides consistent samples and reproducible results, which enables early-stage detection of toxicity in the drug development process.
- In recent years, 3D cell culture models have gained significant traction due to the advantages it offers. For instance, it supports tissue-specific function, differentiation, and increases cell proliferation.
- On the other hand, the OMICS technology segment is estimated to register the highest CAGR over the forecast period as it offers personalized toxicology data, thereby providing better insights and reducing variability in the procured data.
Based on the Application Insights, the market is segmented into Systemic Toxicology, Dermal Toxicity, Endocrine Disruption, Occular Toxicity and Others.
- The systemic toxicology segment dominated the market for in-vitro toxicology testing and accounted for the largest revenue share of over 68.0% in 2020. The development of multiple organ plates with simulated blood flow for assessing systemic toxicity drives the segment growth.
- The dermal toxicity segment is estimated to witness significant growth over the forecast period. Toxicology assays for dermal toxicology have proven to be a simple, reliable, and cost-effective choice.
- The presence of regulatory guidelines for cosmetic toxicity testing drives the demand for in-vitro testing methodologies. The availability of toxicity assays adhering to government guidelines further fuels the segment growth.
Based on the Method Insights, the market is segmented into Cellular Assay, Biochemical Assay, In-Silico and Ex-vivo.
- The cellular assay segment dominated the market for in-vitro toxicology testing and accounted for the largest revenue share of 43.8% in 2020. This can be attributed to the wider availability of this technique for pharmacokinetic profiling of drug products.
- In-vitro cell viability assays offer several advantages, such as minimal cost, speed, and potential for automation. However, these assays pose several technical challenges and are yet to completely replace animal testing, thus posing challenges to their growth.
- Ex-vivo or small explant cultures enable drug screening experiments to be conducted in a sterile, controlled, and dynamic environment by maintaining the native architecture of the tissues.
Based on the End-use Insights, the market is segmented into Pharmaceutical Industry, Cosmetics & Household Products, Academic Institutes and Research Laboratories, Diagnostics, Chemical Industry and Food Industry.
- The pharmaceutical industry segment dominated the market for in-vitro toxicology testing and accounted for the largest revenue share of over 40.0% in 2020.
- To comply with the changing regulatory guidelines for drug development, these assays are mainly used in three areas-ADME, safety pharmacology, and genotoxicity testing of drug candidates. In addition, the development of biologics and biosimilars has resulted in a higher penetration of toxicity tests based on in-vitro models.
- The chemical industry is expected to register lucrative growth over the forecast period owing to ongoing advancements associated with toxicity profiling in this sector. Strategic implementation of these tools for informed and efficient safety assessment at all stages of product development is expected to drive the demand for in-vitro toxicology testing in the industry.
In-vitro Toxicology Testing Regional Outlook
- North America
- Europe
- Asia Pacific
- Latin America
- Middle East & Africa (MEA)
Key Companies Profile & Market Share Insights
This industry shows the presence of a number of sector participants due to which there is an intense internal rivalry to occupy revenue share. Market participants are involved in the development of software solutions in order to enhance in-silico simulation methods for toxicological profiling of different compounds.
Some of the prominent players in the in-vitro toxicology testing market include:
- Merck KGaA
- Charles River
- Bio-Rad Laboratories, Inc.
- Abbott
- Thermo Fisher Scientific Inc.
- Catalent, Inc.
- GE Healthcare
- Quest Diagnostics Incorporated
- Eurofins Scientific
- Laboratory Corporation of America Holdings
- Evotec
- Creative Bioarray
- Gentronix
- BioIVT
- SGS SA
- Agilent Technologies
Order a free sample PDF of the In-vitro Toxicology Testing Market Intelligence Study, published by Grand View Research.
Comments
Post a Comment