Advanced Therapy Medicinal Products CDMO Market Size And Growth Factors By 2028
Advanced Therapy Medicinal Products CDMO Industry Overview
The global advanced therapy medicinal products CDMO market size was valued at USD 5.29 billion in 2020 and is expected to expand at a compound annual growth rate (CAGR) of 12.0% from 2021 to 2028.
The growing demand for advanced therapy is the key factor fueling the market growth. The growth is attributed to the increasing prevalence of rare and life-threatening diseases, such as metabolic and optical diseases, and rising investment in R&D of advanced therapy medicinal products. Besides, ATMPs such as mesenchymal stem cells (MSCs) are a new treatment effective against the COVID-19 virus.
Gather more insights about the market drivers, restrains and growth of the Global Advanced Therapy Medicinal Products CDMO Market
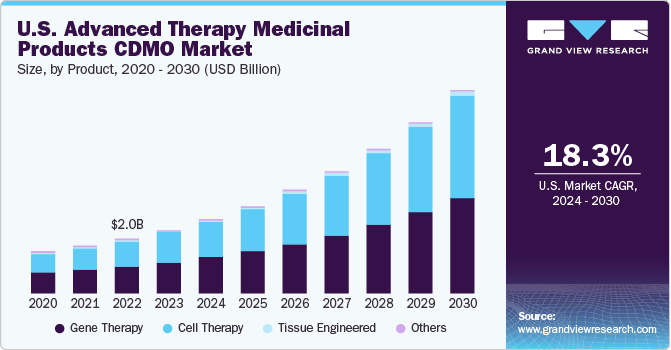
The COVID-19 pandemic has had a massive impact on the development and manufacturing of advanced therapy medicinal products. The number of clinical trials for ATMPs increases due to the efficacy of the product such as MSCs treatment to fight against the virus. The FDA has established a special program for the acceleration of possible therapies for COVID-19 treatment. The program extends support for continuous activity in the clinical trials space and testing of drug molecules for the virus, which will ensure the safety and effectiveness of the drug. Gene and cell therapy have been identified as a potential treatment for the COVID-19 virus, as a result, the regulatory authorities have accelerated the drug development process and supply chain management.
In the last few years, the development of advanced therapy medicinal products has been growing as the demand for the product rises. In 2020, there were around 1,078 ongoing clinical trials of ATMPs globally. With the rising demand for the products and tremendous growth of gene and cell therapy, the role of CDMO has also increased. The biopharmaceutical companies outsource the manufacturing services to CDMOs to focus on the core functions of the organization. CDMO provides a complete package right from the planning for the clinical trial to the actual manufacturing of the drugs. They also help distribute the high cost of research for these products over a period, based on the contracts.
Many organizations choose to install in-house capabilities for the development of the drugs for better control and to save cost but CDMOs remain an integral part of manufacturing the drugs with more amount of experience and expertise as the development of advanced therapy products is a complicated process. CDMOs eventually, however, do charge an exorbitant price to cover their expenses but as a result, they provide high-quality manufacturing services, transparency, and expertise.
Browse through Grand View Research's Medical Devices Industry Research Reports.
- Cell Therapy Market - The global cell therapy market size was valued at USD 7.8 billion in 2020 and is expected to expand at a compound annual growth rate (CAGR) of 14.5% from 2021 to 2028.
- Gene Therapy Market - The global gene therapy market size was valued at USD 2.26 billion in 2020 and is expected to grow at a compound annual growth rate (CAGR) of 20.4% from 2021 to 2028.
Market Share Insights
- November 2020: Catalent has successfully acquired Bone Therapeutics’ cell therapy manufacturing subsidiary. The acquisition will expand the cell therapy capabilities of Catalent allowing them to take up more projects within this space.
Key Companies profiled:
Some prominent players in the global advanced therapy medicinal products CDMO market include
- Celonic
- Bio Elpida
- CGT Catapult
- Rentschler Biopharma SE
- AGC Biologics
- Catalent
- Lonza
- WuXi Advanced Therapies
- BlueReg
- Minaris Regenerative Medicine
- Patheon
Order a free sample PDF of the Advanced Therapy Medicinal Products CDMO Market Intelligence Study, published by Grand View Research.
Comments
Post a Comment