North America Molecular Diagnostics Market 2030: The Rise of Point-of-Care Testing
North America Molecular Diagnostics Market Growth & Trends
The North America molecular diagnostics market size is expected to reach USD 7.23 billion by 2030, registering a CAGR of 4.2% from 2024 to 2030, according to a new report by Grand View Research, Inc. The introduction of technologically advanced products is expected to drive the market in North America. Advancements in molecular diagnostic technology enabled the early detection of numerous diseases and reduced the possibility of severe economic & social burdens. Molecular diagnostics enable early diagnosis of cancer, genetic disorders, and infectious diseases by using PCR, sequencing, & genetic technologies.
The rise in the prevalence of chronic & infectious diseases and genetic disorders, such as Alzheimer’s disease, Turner syndrome, & Parkinson’s disease, is expected to boost the market demand during the forecast period. According to the CDC, chronic diseases such as cancer & diabetes and chronic kidney & respiratory diseases are accountable for 7 in 10 deaths in the U.S. each year. Around 6 out of 10 adults are likely to suffer from a chronic disease in the U.S. In addition, the increasing incidence of diseases, such as influenza A & B, is projected to drive market growth. For instance, according to the CDC, in the flu season of 2021–2022, influenza was estimated to affect nine million people.
Gather more insights about the market drivers, restrains and growth of the North American Molecular Diagnostics Market
An increase in external funding to conduct clinical studies in molecular diagnostics is anticipated to fuel the market. Funding plays a significant role in the product development process. For instance, in April 2023, Promega Corporation announced to grant of USD 15,000 in 2023 in academic life sciences research using qPCR. Similarly, in June 2023, Accelerate Diagnostics, Inc. received USD 24 million to accelerate the development of Wave platform and novel rapid testing. Such initiatives are expected to encourage SMEs to be involved in the development of novel molecular diagnostic tests.
Key companies are making continuous efforts to launch new products in North America molecular diagnostics and gain a competitive edge by meeting the changing needs of buyers toward more advanced & efficient products. For instance, in November 2022, Cepheid, a Danaher subsidiary, launched Multiplex Vaginal Panel (MVP), a PCR test to detect various conditions, such as trichomoniasis, bacterial vaginosis, and vulvovaginal candidiasis.
Furthermore, increasing strategic partnerships among manufacturers and companies for developing novel therapeutics is anticipated to drive market growth. For instance, in February 2023, Thermo Fisher Scientific Inc. partnered with MyLab to procure RT-PCR kits for various infectious diseases, such as tuberculosis & HIV. Moreover, in January 2023, QIAGEN and Helix formed an exclusive alliance to enhance next-generation sequencing companion diagnostics in genetic disorders.
North America Molecular Diagnostics Market Report Highlights
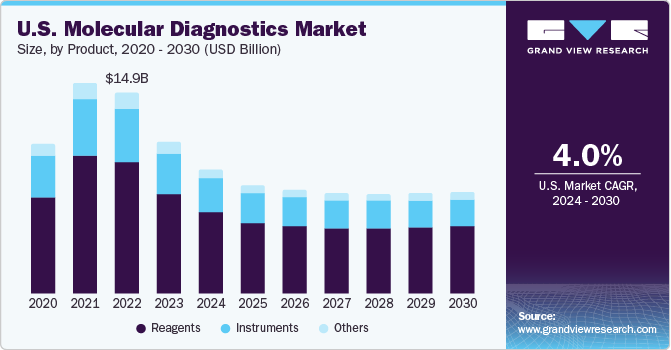
- Based on the product, the reagents segment accounted for the largest revenue share of North America molecular diagnostics in 2023 owing to improved efficiency and cost-effectiveness
- The central laboratories segment dominated the North America molecular diagnostics in 2023 owing to high market penetration and large procedure volumes
- Based on technology, the PCR segment dominated the North America molecular diagnostics in 2023 and is the most preferred technology amongst doctors and patients for clinical diagnostics
- The infectious disease segment led the application segment in 2023, attributable to North America's high incidence of infectious diseases
- The U.S. dominated the regional market due to favorable reimbursement policies, the local presence of leading players, and the presence of well-established healthcare infrastructure
North America Molecular Diagnostics Market Segmentation
Grand View Research has segmented the North America molecular diagnostics market based on product, test location, technology, application, and country:
North America Molecular Diagnostics Product Outlook (Revenue, USD Million, 2018 - 2030)
- Instruments
- Reagents
- Others
North America Molecular Diagnostics Test Location Outlook (Revenue, USD Million, 2018 - 2030)
- Point-of-Care
- Self-test or Over the Counter
- Central Laboratories
North America Molecular Diagnostics Technology Outlook (Revenue, USD Million, 2018 - 2030)
- Polymerase Chain Reaction (PCR)
- In Situ Hybridization
- Isothermal Nucleic Acid Amplification Technology (INAAT)
- Chips and Microarrays
- Mass spectroscopy
- Sequencing
- Transcription Mediated Amplification (TMA)
- Others
North America Molecular Diagnostics Application Outlook (Revenue, USD Million, 2018 - 2030)
- Oncology
- Pharmacogenomics
- Infectious Diseases
- Genetic Testing
- Neurological Disease
- Cardiovascular Disease
- Microbiology
- Others
North America Molecular Diagnostics Regional Outlook (Revenue, USD Million, 2018 - 2030)
- North America
- U.S.
- Canada
Order a free sample PDF of the North American Molecular Diagnostics Market Intelligence Study, published by Grand View Research.
Comments
Post a Comment