Glioblastoma Multiforme Treatment Market Threats, Challenges, Competitive Scenario 2028
Glioblastoma Multiforme Treatment Industry Overview
The global glioblastoma multiforme treatment market size was valued at USD 2.14 billion in 2020 and is expected to expand at a compound annual growth rate (CAGR) of 8.8% from 2021 to 2028.
The growing prevalence of glioblastoma multiforme, increasing R&D, and favorable regulatory scenarios are among the factors anticipated to fuel the market growth. The presence of a strong pipeline is expected to act as a major driver for the glioblastoma multiforme (GBM) treatment market during the forecast period. The increasing incidence of brain tumors is expected to boost the growth of the market over the forecast period. According to Global Cancer Observatory, in 2020, the incidence of brain and CNS cancers was 308,102 globally and the number of deaths was 251,329 worldwide. Brain cancer is one of the deadliest forms of cancer.
Gather more insights about the market drivers, restrains and growth of the Global Glioblastoma Multiforme Treatment Market
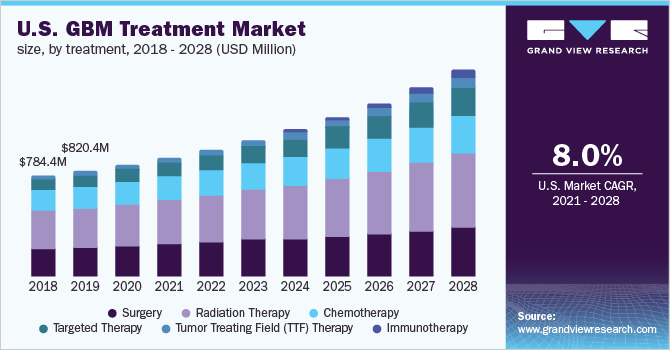
Glioblastoma multiforme is the most common form of malignant tumor, accounting for up to 54% of gliomas and 16% of all primary brain cancers. The incidence of glioblastoma multiforme is high in adults compared to children and the risk of developing glioblastoma multiforme increases with age.
The increasing approval for novel therapy and combination therapy is expected to drive the market in the coming years. For instance, in June 2019, the U.S. FDA approved Pfizer’s Zirabev, a biosimilar for Avastin, for the treatment of recurrent glioblastoma, NSCLC, and colorectal cancer, among others. In January 2020, the company launched its product in the U.S. Moreover, in November 2019, the U.S. FDA accepted Samsung’s BLA application for its SB8 bevacizumab biosimilar candidate, which will increase the potential to launch products in the coming years.
Tumor heterogeneity and variation in the patient-to-patient treatment approach are expected to contribute to an increase in the demand for a personalized treatment approach to manage glioblastoma multiforme. The approval of new treatments is expected to increase the life expectancy of patients living with glioblastoma multiforme. Furthermore, a special designation granted to investigational drugs by the FDA is expected to expedite the approval process and commercialization of novel therapy. For instance, in July 2020, the U.S. FDA granted fast track designation to Denovo biopharma’s DB102 (enzastaurin) for the treatment of patients newly diagnosed with glioblastoma.
Browse through Grand View Research's Pharmaceuticals Industry Research Reports.
- Excipients Market: The global excipients market size was estimated at USD 5.53 billion in 2021 and is expected to advance at a compound annual growth rate (CAGR) of 6.0% from 2022 to 2030.
- Inhalation Anesthesia Market: The global inhalation anesthesia market size was valued at USD 1.1 billion in 2021 and is projected to expand at a CAGR of 9.0% in the forecast period.
Market Share Insights
- April 2021: Lineage Cell Therapeutics entered into a licensing agreement with Immunomic Therapeutics for the development of a cancer immunotherapy platform, allogeneic VAC, to treat glioblastoma multiforme.
- December 2017: The company received full approval from the U.S. FDA for bevacizumab for the treatment of adults with GBM.
Key Companies profiled:
Some of the prominent players in the global glioblastoma multiforme treatment market include:
- Merck & Co., Inc.
- Amgen, Inc.
- Hoffmann-La Roche Ltd.
- Pfizer Inc.
- Amgen, Inc.
- Teva Pharmaceutical Industries Ltd.
- Sun Pharmaceutical Industries Ltd.
- Arbor Pharmaceuticals, LLC
- Amneal Pharmaceuticals
- Karyopharm Therapeutics, Inc.
- Sumitomo Dainippon Pharma Oncology, Inc. (Boston Biomedical, Inc.)
Order a free sample PDF of the Glioblastoma Multiforme Treatment Market Intelligence Study, published by Grand View Research.
Comments
Post a Comment